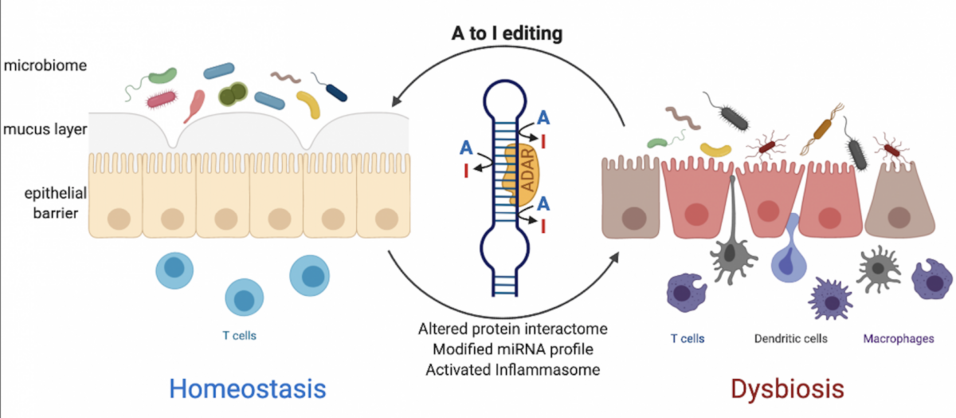
Editing is essential in mice, as deletions of the responsible deaminases, ADAR1 or ADAR2, are lethal. Inosine is mostly read as guanosine (G), hence editing can recode messenger RNA (mRNA) impacting the protein sequence. However, the precise role of particular RNA modifications in health and disease remains to be elucidated.
Recent epidemiological data estimates that 2.5-3 million people in Europe are affected by either Crohn´s disease or ulcerative colitis, the two main forms of IBD, with a direct healthcare cost of 4.6-5.6 bn Euros/year. IBDs result from an exacerbated response of the host immune system to a dysbiotic gut microbiota in genetically predisposed individuals. Whether dysbiosis is the cause or consequence of IBD is debatable, but IBD response to antibiotics supports a key role of microbiota in pathogenesis and disease progression. In the gut, host and microbiota constantly communicate, and RNA moieties such as microRNAs (miRNAs) are important for such crosstalk. MiRNAs are small non-coding RNAs that can bind to target mRNAs, leading to translation inhibition or transcript degradation. Strikingly, the expression of several miRNAs is altered in IBD and other chronic inflammatory diseases. Furthermore, depletion of particular fecal miRNAs causes shifts in microbiota composition and alters mouse susceptibility to colitis in a microbiota-dependent fashion. Despite the clear involvement of miRNAs in IBD and gut microbiota modulation, the impact of luminal miRNAs and miRNA editing in the large intestine has not been thoroughly investigated.
Imbalanced immune homeostasis of the gut, influenced by microbiota dysbiosis and genetic predisposition, is at the basis of IBD. One of the central homeostatic mechanisms to limit aberrant interferon (IFN) responses is discrimination of self from non-self RNA by RNA editing. Editing of cellular dsRNA prevents recognition of self RNA as a danger signal. Organism-wide, RNA-editing can suppress inflammation by inhibiting the RNA helicase MDA5. Potentially, also other MDA5 related helicases are involved in modification-dependent protection from hyperinflammation.
The central hypothesis of this project is that RNA editing is an integral part of vital regulatory networks like miRNA-dependent gene regulation, adjustment of relevant protein interactomes and the control of aberrant immune stimulation and is therefore a key determinant in dysbiosis, inflammation and cancer. To tackle this we combine “omic” approaches (metagenomics and transcriptomics), state-of-the-art mouse models of IBD, microbial ecology cultivation-dependent and -independent techniques and a completely novel tissue-specific editing approach by CRISPR/Cas9. We aim to investigate in detail: 1) how editing of particular mRNAs impacts IBD; 2) the impact of fecal microRNAs and miRNA editing on the host-microbiota crosstalk; 3) the role of helicases in discriminating self vs. non-self RNA. Results will advance the field of epitranscriptomic research and potentially serve as a basis to diagnose or treat IBD and colon cancer.
This project in funded by the Austrian Science Fund (Project ZK-57).
Team:
Cornelia Vesely (Principal Investigator, Coordinator), Division of Cell and Developmental Biology, Center for Anatomy and Cell Biology, Medical University of Vienna.
Riem Gawish (Principal Investigator), Research Laboratory of Infection Biology, Department of Medicine 1, Medical University of Vienna; Research Center for Molecular Medicine of the Austrian Academy of Sciences (CeMM).
Krzysztof Chylinski (Principal Investigator), Genome Engineering Unit at Protein Technologies Facility, Vienna Biocenter Core Facilities GmbH.
Fatima Pereira (Principal Investigator), Division of Microbial Ecology, Centre for Microbiology and Environmental Systems Science, University of Vienna.
Daniel Crepaz (Bachelor student), Division of Microbial Ecology, Centre for Microbiology and Environmental Systems Science, University of Vienna
Salwan Roumaia (Technical assistant), Division of Cell and Developmental Biology, Center for Anatomy and Cell Biology, Medical University of Vienna.
Jurgita Ozelyte (Master Student), Division of Microbial Ecology, Centre for Microbiology and Environmental Systems Science, University of Vienna.
Investigated by: