Dr. Marc Mußmann
Marc’s research investigates the valuable ecosystem service provided by marine coastal sediments as biocatalytic filters. As a PhD and postdoctoral researcher at the Max Plank Institute Bremen (Germany), he studied the role of microbes in breaking down organic matter and pollutants, such as hydrogen sulfide. Currently, he investigates the function of the globally abundant bacterial family Woeseiaceae in marine sediments using various molecular techniques. Recently, he has discovered the presence of electroactive bacteria in marine sediments that thrive on minerals as “batteries.” He also develops novel methods to identify and study macromolecule-degrading microbes in diverse habitats. To support research at the UBB, he provides training and support in flow cytometry.
Links

Main Research Interests
1. Biology and ecogenomics of the bacterial taxon Woeseiales, key players in global marine sediments
(FWF-funded 2018 - 2022, project # P31010 )
These bacteria are tiny but account for more than 10 million cells in 1 g of beach sand. Because of their ubiquity and their high cell numbers they are expected to substantially impact element cycles in sediments worldwide (Mußmann et al. 2017). It is thus counterintuitive that the Woeseiaceae can hardly be grown and maintained in the laboratory to study their biology in detail. In this project we systematically study the function of Woeseiaceae in nature. We apply modern genetic tools, sequencing of DNA and RNA recovered from the seafloor, which promises unprecedented insights into their actual function and why these bacteria are so successful in marine sediment and what they live from. First results suggest that the Woeseiaceae live well on sugars and proteins, while breathing oxygen and CO2 - just like human beings. But Woeseiaceae are special: it appears that some thrive also on hydrogen, sulfur, nitrite and rusty iron.
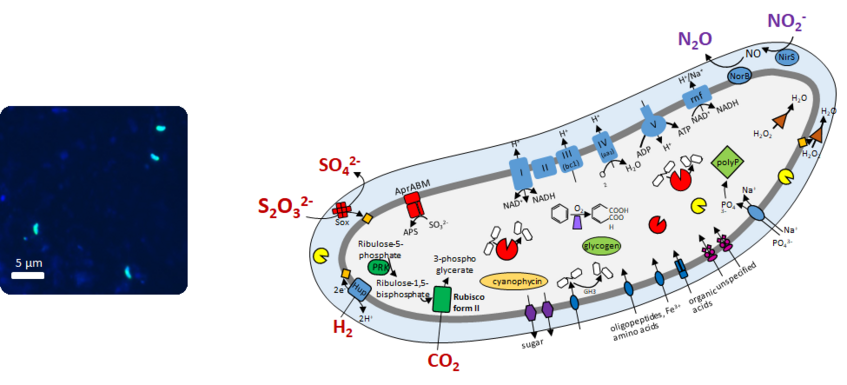
Mußmann et al. (2017, ISME J)
2. Iron-cycling microorganisms in coastal surface sediments
Marine sediments contain substantial amounts of magnetic iron, mostly magnetite (Fe3O4). We investigate, whether and how microbes (e.g., the Woeseiaceae) use this as energy source for oxidation, as electron acceptor for respiration or as a rechargeable electron reservoir. Using strong magnets we pull out magnetic particles from diverse coastal sediments and study the microorganisms specifically colonizing particulate iron.

3. H2-consuming and -producing bacteria in intertidal sediments
Molecular hydrogen (H2) is an universal key intermediate for microbial metabolism in basically all anoxic but also oxic habitats including aquatic sediments, soil and gastrointestinal tracts. Main sources of H2 in the ocean are processes such as phototrophy, N2-fixation and in particular anaerobic fermentation in the seafloor. Despite the high relevance of fermentative H2 production, the contributing organisms are largely unknown. Interestingly, some members of the family Woeseiaceae posses genes for both H2 production and consumption. Here, we study the community composition of actively H2-cycling by novel growth-stimulating techniques techniques. These enrichments are then tested for their H2-cycling potential and (meta)genome sequenced.
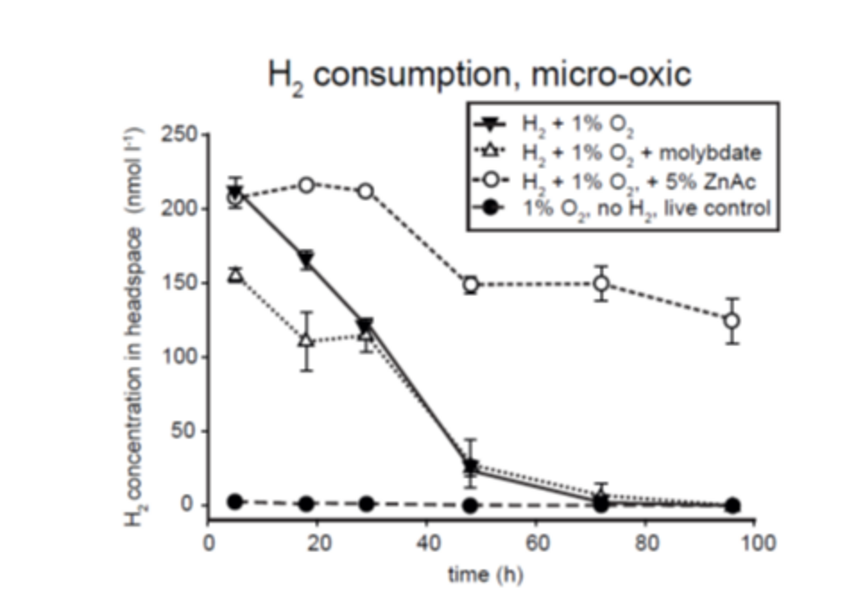
Dyksma et al. (2018)
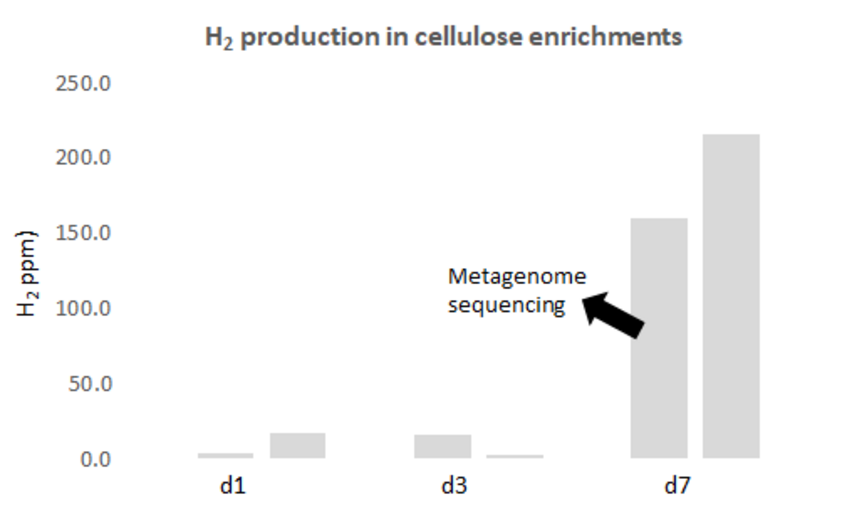
unpublished data
4. Microbial sulfur oxidation in intertidal sediments
Toxic sulfide may have caused several mass extinctions of terrestrial and oceanic life in the history of Earth. Its oxidation was a condition for oxygen-breathing life as we know it today. Today, most sulfide is already oxidized at marine sediment surfaces, but it can still cause increasing ecological and socioeconomic damages in coastal areas. Considering the predicted expansion of sulfidic zones in future oceans, it is imperative to decipher the underlying mechanisms of sulfide detoxification in marine sediments. The current view of sulfide oxidation in sediments is biased towards chemical processes, while the general role of microbial sulfide oxidation in vast areas of the seafloor is unknown. However, modeling suggests that biological far outcompetes chemical sulfide oxidation in most environments. Consistent with this we recently identified myriads of novel, potentially sulfide-oxidizing bacteria (“sulfur oxidizers”) in sediments worldwide (Dyksma et al., 2016, Mußmann et al., 2017). Through their ability to fix CO2, they may also trap carbon in amounts that could significantly attenuate seafloor CO2 emissions. Although sulfur oxidation is essential to ecosystem functioning, there are surprisingly large gaps in our basic understanding of these processes. Here, we study the physiology and significance of this overlooked majority of sulfur oxidizers in marine sediments using metagenomics and -transcriptomics.

Methods
- The full 16S rRNA cycle, fluorescence in situ hybridization (FISH), CARD-FISH
- Metagenomics, -transcriptomics
- Isotope probing (13C, 14C, 15N) in single cells and populations, nanoSIMS
- Bioorthogonal Noncanonical Amino Acid Tagging (BONCAT)
- Novel in situ enrichment techniques
- Metabolomics
- Flow cytometry and cell sorting
Former lab members

Former lab members: Clara Martinez-Perez, Marc Mußmann, our site host Helge-Ulrike Harms, Isabella Hinger, Claus Pelikan (Utah Beach, France 2019)

