Despite this, we so far understand the underlying mechanisms in very few systems. This knowledge is the basis for manipulating microbiomes for better environmental, animal, and human health. There is still immense potential for discovering fundamentally new mechanisms of host-microbe interaction among the staggering diversity of animals and their microbial symbionts in nature. This project aims to use the ancient and exclusive association between marine lucinid clams and chemosynthetic symbiotic bacteria to investigate fundamental aspects of host-microbe interactions.
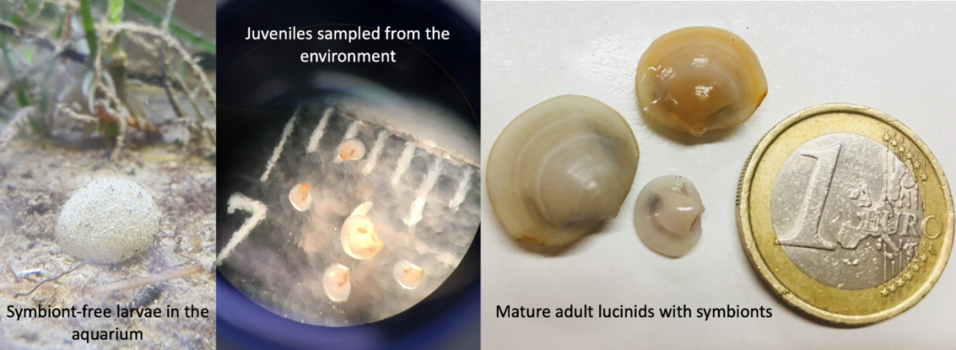
Lucinidae is one of the most widespread and species-rich animal families in the oceans today, and has lived in symbiosis for more than 400 million years. The clam’s outstanding ability to select one specific symbiont from the trillions of bacteria in its environment challenges widely held assumptions about the function and specificity of the innate immune system. Symbiont-free juveniles can be raised in the lab, and experimentally infected, allowing unmatched insights into the early development of this symbiosis. Although the symbiont infection is specific to gill cells, symbiont-encoded proteins can be found in distant parts of the animal that are symbiont-free. This project combines cutting-edge molecular tools and experimental infection to better understand three key aspects of host-microbe interactions in these clams: 1) Acquisition and selection of microbes during animal development, 2) Maintenance along animal lifetimes through molecular communication and exchange, and 3) Emergence and perpetuation over evolution. I hypothesize that intracellular bacterial symbionts fundamentally alter host biology, and these effects are not limited to the location where symbionts are housed, but can affect distant organ systems. The overarching goal is to understand the molecular basis for these effects, and their evolutionary history.
Our efforts are aimed at establishing a new experimental system for understanding fundamental aspects of host-microbe interactions. This system goes beyond the state of the art as it would be the first model of animal symbiosis where the symbionts invade the host cells from the environment.
Host-microbe interactions are the basis for animal (including human) health and disease. A better understanding of the molecular underpinnings of these processes will help us to understand how these interactions function, and how they might have evolved. This understanding is of fundamental importance for a wide array of biological, environmental, and medical research fields.
This project is funded by a European Research Council (ERC) Starting Grant (802494).